101
Tecnología y Ciencias del Agua
, vol. VIII, núm. 2, marzo-abril de 2017, pp. 93-103
Song & Song,
Kinetics and influential factors of nanoscale iron-facilitated nitrate-nitrogen removal
ISSN 2007-2422
•
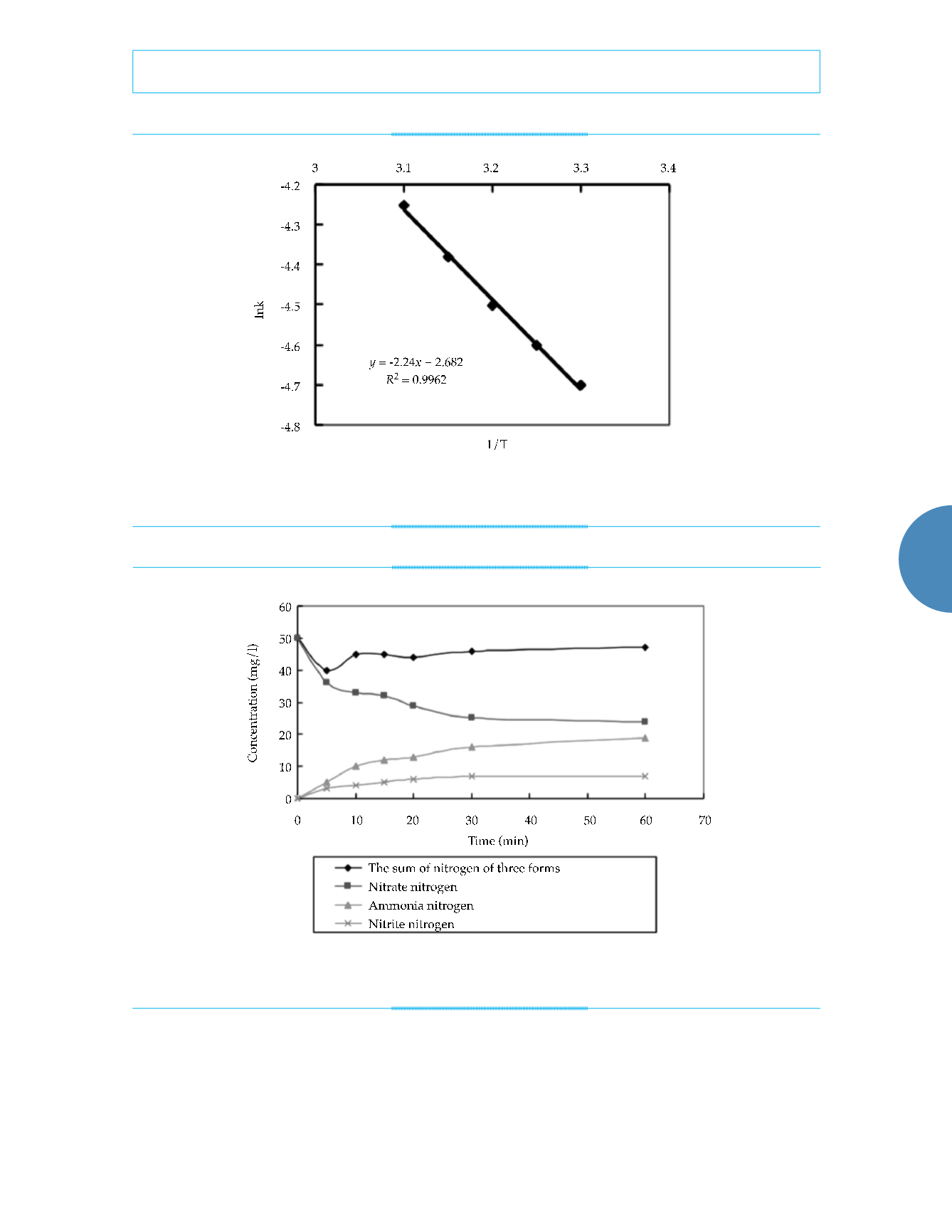
nitrogen was converted to ammonia and
nitrite nitrogen, which were later removed.
As shown in equations (5) and (6), under the
neutral reaction conditions, most of the nitrate
nitrogen was converted to ammonia. Thus,
the nitrate nitrogen was primarily reduced to
ammonia. The standard electrode potential of
the Fe
0
and its oxidation-reduction reaction
with Fe
2+
within the liquid solution (Fe
0
/Fe
2+
)
was approximate -0.440 V, indicating that Fe
0
Figure 6. Analysis of the activation energy required to initiate nitrate-nitrogen removal by nanoscale iron.
Figure 7. Production of nitrate´s deoxidization by nanoscale iron (at a constant temperature and pH of 25 ˚C and 7.0.).


