99
Tecnología y Ciencias del Agua
, vol. VIII, núm. 2, marzo-abril de 2017, pp. 93-103
Song & Song,
Kinetics and influential factors of nanoscale iron-facilitated nitrate-nitrogen removal
ISSN 2007-2422
•
reaction occurs. Thus, there is no universally ac-
cepted kinetic equation for the reaction between
nanoscale iron and nitrate nitrogen. In this
paper, a pseudo-second-order kinetic equation
was developed in order to determine the value
of the reaction rate constant at different tem-
peratures. Since the nitrate removal rate in this
study ranged from 50 to 60%, not appropriate to
research its kinetics equation from the half-life
point of view. The data also indicated that the
reaction became constant after 10 minutes due
to the increased concentration of reacted nitrate
nitrogen and resulting decrease in the reaction
rate. The following pseudo-second-order kinetic
equation was used to describe the reaction be-
tween the nitrate nitrogen and nanoscale iron:
d
C
i
/
d
t
=
k
(
C
a
-
C
i
)
2
(6)
In this equation,
C
i
represents the difference
between the initial nitrate concentration C
0
and
the nitrate concentration at time
t C
t
(mg/l),
C
a
represents the difference between the initial
nitrate concentration
C
0
and the stable nitrate
concentration
C
e
(mg/l), and
k
is the reaction
rate constant (mg/(l·min). This equation was
integrated and simplified as:
C
t
=
C
0
-
k
t
C
2
a
/1+
k
t
C
a
(7)
Equation (7) was used to calculate the value
of
k
. Then, the appropriate value of
k
was se-
lected based on the statistical error between the
fitted value and experimental data. The results
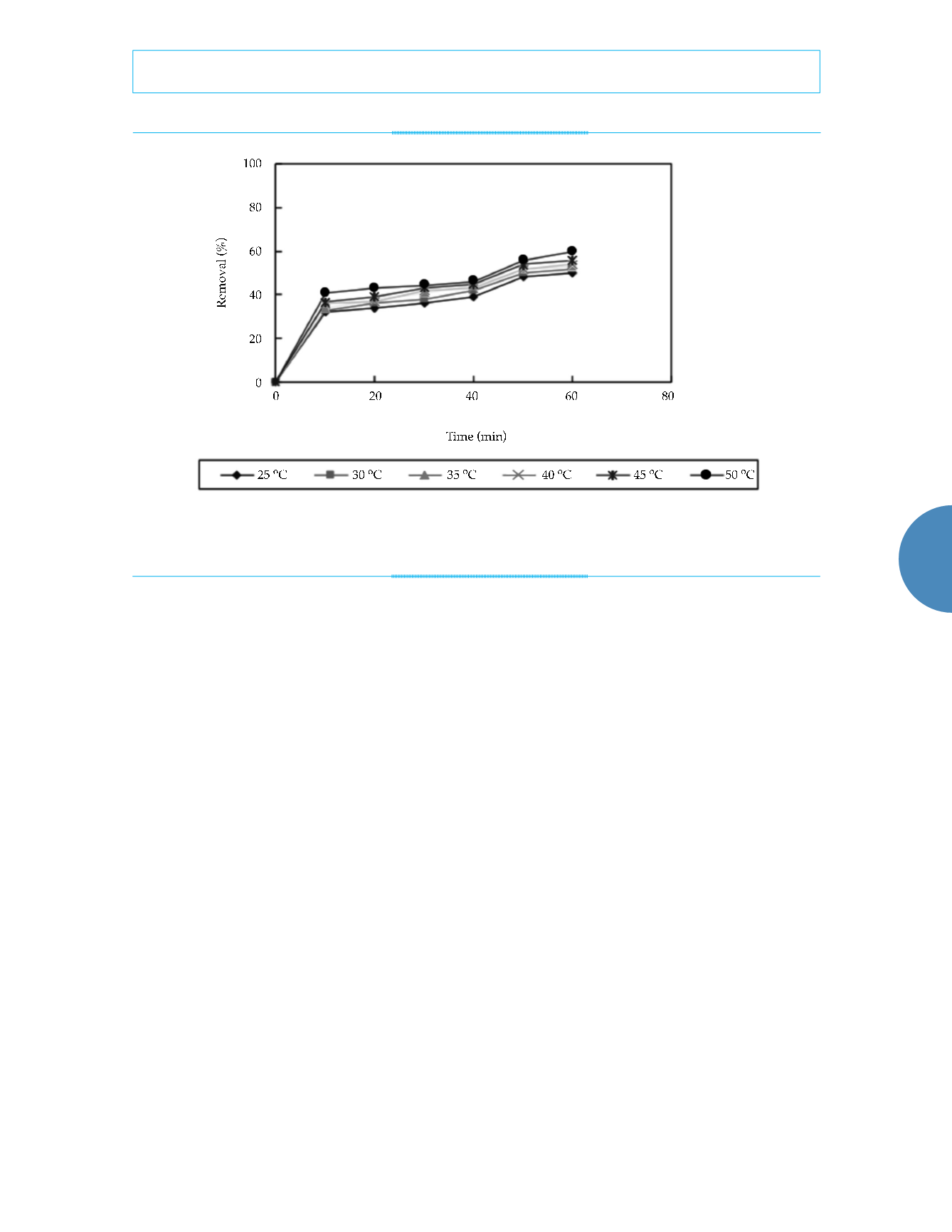
are shown in figure 5 and table 1.
Temperature also affects the reaction rate.
According to the Arrhenius equation:
In
k
=
Ea
/
RT
+ In
A
(8)
An lnk 1/T relationship diagram was con-
structed in order to determine the activation
energy, as shown in figure 6. According to the
data presented in figure 6, the activation energy
Ea
of the reaction was approximately 17.18 kJ/
mol. First, the nitrate nitrogen was absorbed by
the nanoscale iron. Next, the nitrate nitrogen
reacted with the surface of the nanoscale iron.
Then, a fraction of the zero-valent nanoscale iron
was converted into divalent iron ions. Therefore,
Figure 4. Influence of different temperature on removal efficiency (in the experiment, 0.5 g of nanoscale iron was added to water
containing 50 mg/l of nitrate-nitrogen).


