147
Qin
et al
.,
Comparison on nitrosation and anaerobic ammonium oxidation between activated sludge and biofilm from an autotrophic...
Tecnología y Ciencias del Agua
, vol. VIII, núm. 2, marzo-abril de 2017, pp. 141-149
ISSN 2007-2422
•
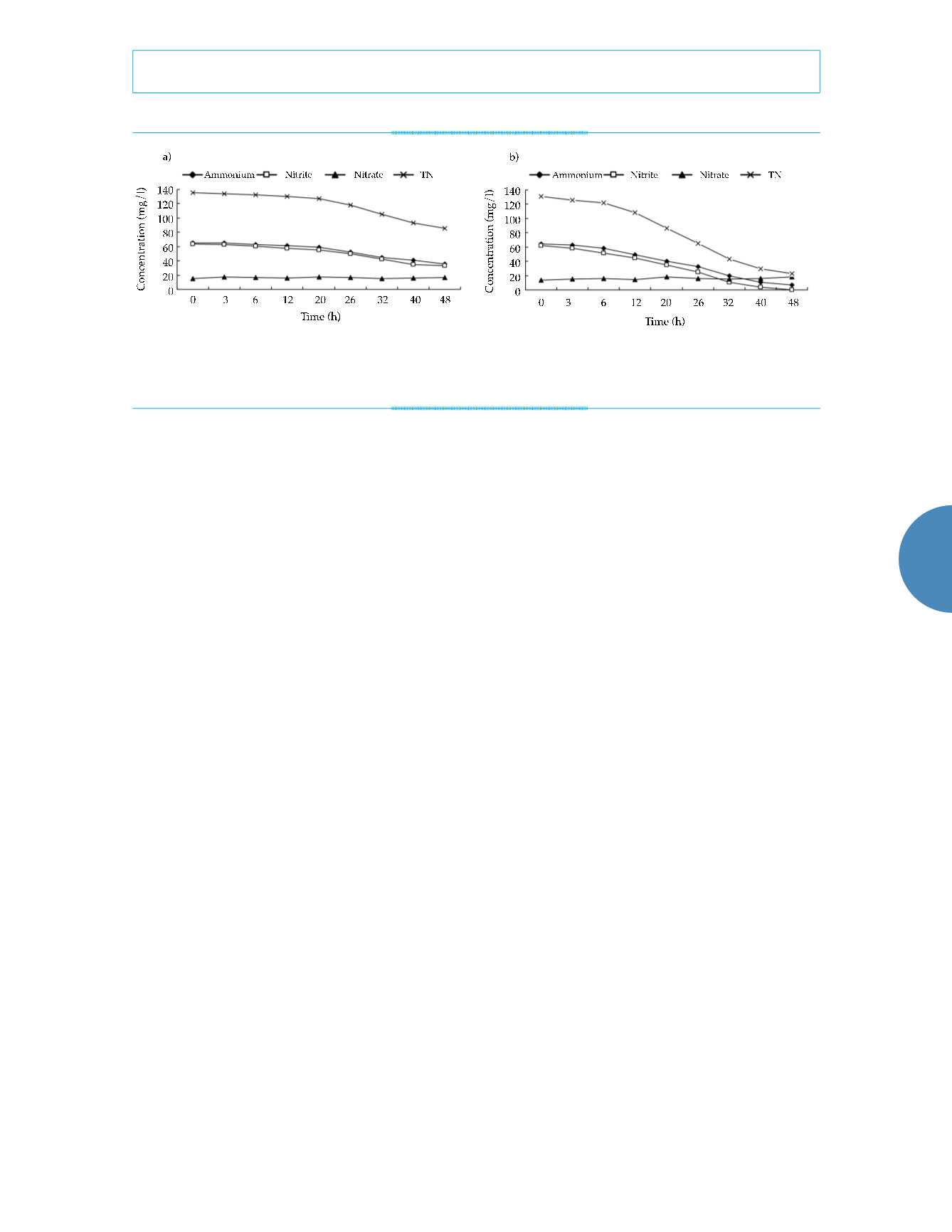
biofilm samples. Initial concentrations of NH
4
+
and NO
2
-
in the artificial wastewater were both
70 mg/l. TN concentrations in both samples
declined over the 48h experimental period. TN
removal rates were 37% by activated sludge
and 83% by biofilm. In contrast with nitrosation
activity, it was confirmed that TN was removed
mainly by anaerobic ammonium oxidation in
this reactor. The reaction rate of anaerobic am-
monium oxidation by activated sludge was 0.09
mgN mgVSS
−1
d
−1
. This was just 41% of the rate
seen in biofilm, which was 0.22 mgN mgVSS
−1
d
−1
.
The concentration of NO
3
-
in the activated
sludge treatment varied between 15.0 and 17.4
mg/l, and in the biofilm treatment, the range
was 13.8 to 18.0 mg/l, though no NO
3
-
-N was
added to the artificial wastewater. The initial
concentrations of NO
3
-
in activated sludge and
biofilm treatments were 15.0 and 13.8 mg/l,
respectively. One possibility is that NaNO
2
-
was
unstable and was partially oxidized to NaNO
3
,
which provided the initial NO
3
-
. Figures 4a and
4b show that the concentrations of NO
3
-
did not
vary greatly over the course of the experiments.
The small fluctuations that were observed may
have been caused by measurement error. Van de
Graaf
et al
. (1995) concluded that ANAMMOX
could utilize both NO
3
-
and NO
2
-
in the anaero-
bic ammonium oxidation reaction because both
can function as electronic receptors to convert
the remaining NH
4
+
into N
2
. When the substrate
contained both NO
3
-
and NO
2
-
, it was the NO
2
-
that acted as the main electronic receptor for
ANAMMOX. In this study, the removal efficien-
cy of TN was high and the largest consumption
rates of NH
4
+
and NO
2
-
in activated sludge were
0.05 and 0.06 mgN mgVSS
−1
d
-1
, respectively
while for biofilm the values were 0.12 and 0.14
mgN mgVSS
−1
d
−1
. The concentration of NO
3
-
in
the system was more-or-less constant, which
suggests that NO
2
-
was the main electronic
receptor in anaerobic ammonium oxidation in
this SBBR system. This was in accordance with
Van de Graaf’s findings.
Conclusions
Activated sludge and biofilm played different
roles in autotrophic nitrogen removal process.
Both activated sludge and biofilm samples
exhibited nitrosation activity in the SBBR au-
totrophic denitrification system, the maximum
ammonia oxidation rates of activated sludge
and biofilm were 0.23 and 0.08 mgN mgVSS
−1
d
−1
, respectively. The nitrosation activity of
activated sludge was about 2.89 times that of
the biofilm. The populations of AOB in activated
sludge and biofilm were 1.88 × 10
11
and 1.90 ×
10
10
cells/g, respectively.
The maximum anaerobic ammonia oxidation
rate of activated sludge was 0.09 mgN mgVSS
−1
d
−1
, just 41% of the maximum rate of biofilm,
which was 0.22 mgN mgVSS
−1
d
−1
. Anaerobic
ammonia oxidation occurred mainly in the
biofilm, with ANAMMOX being the dominant
Figure 4a. Anaerobic oxidation of ammonium. Figure 4b. Anaerobic oxidation of ammonium by activated
sludge by biofilm.


